
.png)

Discover how researchers at TU have achieved a groundbreaking feat in cartilage regeneration by growing cartilage from stem cells.
.png)
Imagine a world where injuries are no longer a setback, but an opportunity for customized repair. Thanks to the groundbreaking research conducted by the dedicated team at TU, that world may soon become a reality. By harnessing the power of stem cells, these researchers have made incredible strides in growing cartilage, offering hope for a brighter future in injury treatment and rehabilitation.
Before we dive into the groundbreaking research at TU, let's take a moment to understand what stem cells are and why they hold so much promise in the field of medical science.
Stem cells are unique cells in our body that have the remarkable ability to develop into different types of specialized cells. They act as the body's natural, built-in repair system, constantly replenishing and regenerating damaged tissues.
Stem cells are undifferentiated cells that have the potential to develop into various types of cells, such as muscle cells, bone cells, or even nerve cells. They can be derived from various sources, including embryos, adult tissues, and even from your own body's fat stores.
Embryonic stem cells, for example, are obtained from embryos that are just a few days old. These cells are pluripotent, meaning they have the ability to differentiate into any type of cell in the body. Adult stem cells, on the other hand, are found in various tissues throughout the body and are multipotent, meaning they can differentiate into a limited number of cell types.
In our bodies, stem cells play a vital role in repairing and regenerating damaged tissues. They contribute to the natural healing process by replenishing cells that have been lost due to injury, disease, or normal wear and tear.
For example, when you cut your finger, stem cells rush to the site of injury and begin dividing and differentiating into the specific types of cells needed to repair the damaged tissue. This process ensures that the wound heals properly and that you regain full function of your finger.
Stem cells have captured the attention of researchers and medical professionals due to their potential to revolutionize the field of medicine. They hold the key to unlocking new treatments for numerous conditions, from heart disease to spinal cord injuries.
With their ability to differentiate into specialized cells, stem cells can potentially be used to grow replacement tissues and organs, offering hope to patients awaiting transplants and reducing the risk of rejection by the body's immune system.
Furthermore, stem cells have the potential to be used in drug discovery and testing. By creating specialized cells from stem cells, researchers can study how diseases develop and test the effectiveness of new drugs in a controlled environment.
While there is still much research to be done and ethical considerations to be addressed, the potential of stem cells in medical science is truly remarkable. As scientists continue to unravel the mysteries of these incredible cells, we can look forward to a future where previously incurable diseases become manageable and where regenerative medicine becomes a reality.
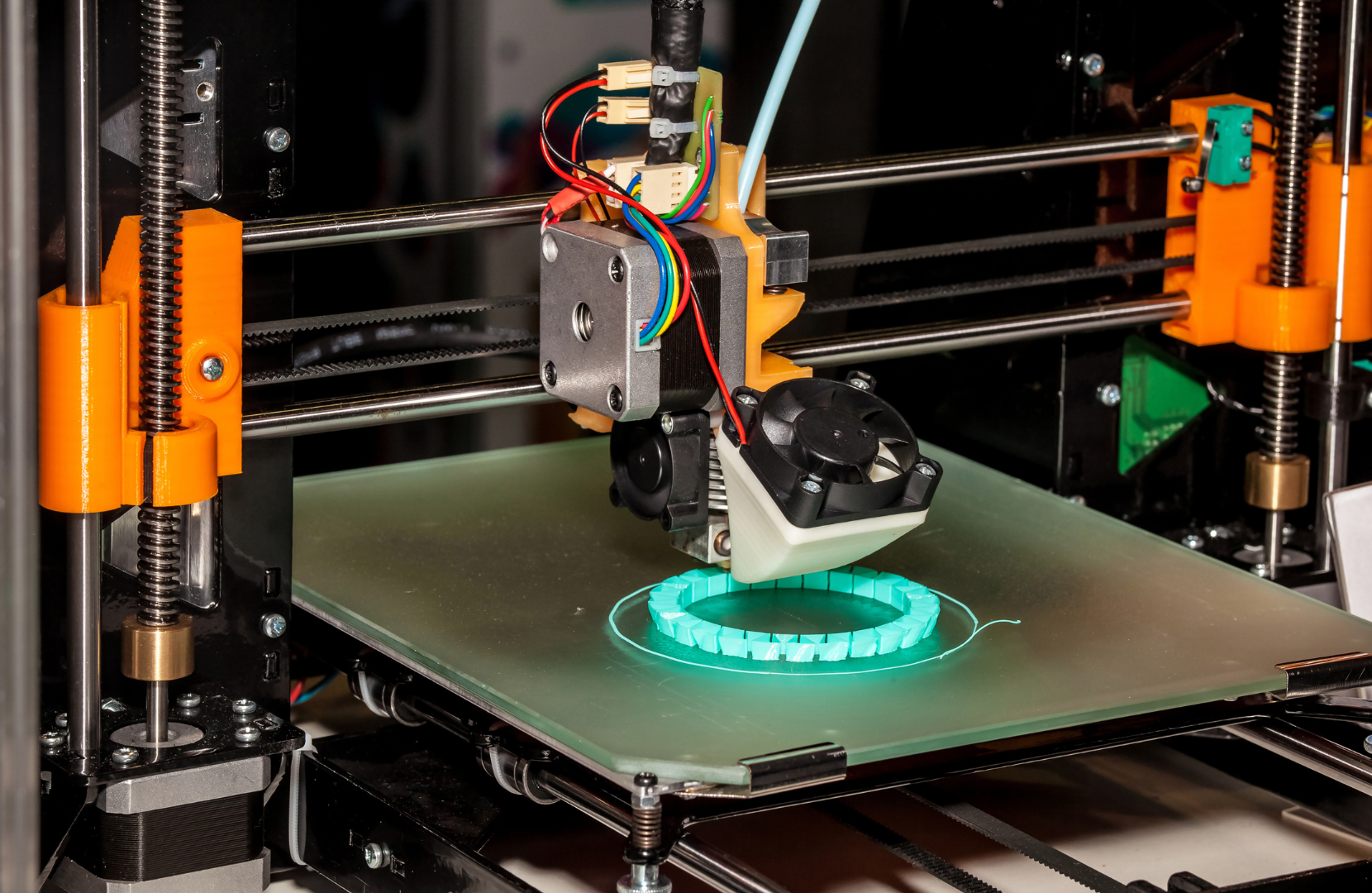
A new approach to producing artificial tissue has been developed at TU Wien: Cells are grown in microstructures created in a 3D printer.
The breakthrough in creating artificial tissue using a 3D printing approach at TU Wien involved a team of researchers from different disciplines working together to develop and implement the new method.
The team was led by Prof. Aleksandr Ovsianikov, who heads the 3D Printing and Biofabrication research group at TU Wien. Oliver Kopinski-Grünwald, from the Institute of Materials Science and Technology at TU Wien, was one of the authors of the study and played a crucial role in the project. Additionally, the team likely included experts in materials science, bioengineering, tissue engineering, and possibly other related fields.
These researchers collaborated closely to design and optimize the 3D printing process, develop the biocompatible and degradable plastic materials, culture the cells, and analyze the resulting tissue constructs. Their interdisciplinary approach and collective expertise were instrumental in achieving this significant advancement in tissue engineering technology.
According to the study conducted at TU Wien, the process of growing cartilage from stem cells involves a novel approach that utilizes high-resolution 3D printing technology. Initially, tiny porous spheres are created using a special 3D printing process with biocompatible and degradable plastic materials. These spheres, resembling miniature footballs with a diameter of just a third of a millimeter, serve as scaffolds for the cells. This unique scaffold design is crucial for providing mechanical support and maintaining the shape of the tissue constructs.
Stem cells, specifically differentiated stem cells predetermined to form cartilage tissue, are introduced into these football-shaped mini-cages. The small size of the cages ensures that the cells evenly fill the volume, resulting in tissue elements with a very high cell density. This approach addresses a common challenge in tissue engineering, where controlling the shape and distribution of cells within the tissue construct is difficult to achieve using conventional methods.
One of the key innovations of this method is the ability to assemble the tissue elements into any desired shape. This flexibility allows researchers to create complex tissue structures tailored to specific applications. Moreover, the study demonstrates that neighboring tissue elements, or spheroids, seamlessly grow together as the cells migrate between them. This seamless integration results in a uniform and homogeneous tissue structure without any visible interfaces between neighboring cell clusters, which is a significant improvement over existing methods.
The 3D-printed porous scaffolds provide mechanical stability to the tissue structure while the cells continue to mature. Over time, the plastic structures degrade, leaving behind the finished tissue in the desired shape. This gradual degradation process ensures that the artificial tissue integrates smoothly with the surrounding tissue environment. Overall, the TU Wien study presents a promising approach for growing cartilage tissue from stem cells using advanced 3D printing technology, with potential applications in regenerative medicine and tissue engineering.
As with any groundbreaking research, the path to success is not without its challenges. The team at TU has faced numerous obstacles, ranging from technical hurdles to ethical considerations.
One of the main challenges addressed by the study is the difficulty in controlling the shape and distribution of cells within tissue constructs. Traditional methods often result in tissue with unpredictable shapes and uneven cell distribution, limiting their suitability for specific applications such as cartilage replacement.
By utilizing high-resolution 3D printing technology, the research team overcomes this challenge by creating precise, miniature scaffold structures that serve as templates for cell growth. These scaffolds provide mechanical support and maintain the shape of the tissue constructs, addressing a critical limitation of conventional tissue engineering methods.
Another challenge in tissue engineering is achieving seamless integration between tissue elements to form a uniform and homogeneous structure. The TU Wien study successfully demonstrates that neighboring tissue elements, or spheroids, grow together seamlessly as the cells migrate between them. This achievement represents a significant triumph in the field, as it overcomes a major obstacle in creating functional artificial tissue.
Additionally, the study highlights the importance of utilizing differentiated stem cells predetermined to form specific tissue types, such as cartilage tissue. This approach ensures that the cells are already committed to a particular lineage, improving the efficiency and effectiveness of tissue formation.
However, despite these triumphs, several challenges remain to be addressed. One such challenge is the incorporation of blood vessels into larger tissue constructs, which is necessary to support the viability and functionality of the tissue over time. Additionally, further research is needed to optimize the 3D printing process and scaffold design for different types of tissue and applications.
The implications of growing cartilage from stem cells are immense, opening doors to a new era of injury repair and rehabilitation.
Imagine a future where injuries are no longer treated with a one-size-fits-all approach. Customized injury repair made possible by stem cells offers the potential to tailor treatments specifically to the needs of each individual, ensuring optimal outcomes.
Picture this: a patient with a knee injury walks into a clinic. Instead of receiving a generic treatment plan, their injury is assessed, and a personalized approach is developed. Stem cells are harvested from the patient's own body and used to grow cartilage that perfectly matches their unique physiology. This customized approach not only enhances the effectiveness of the treatment but also minimizes the risk of complications or rejection.
By harnessing the power of stem cells, researchers could unlock new treatment options for a wide range of injuries, including damaged cartilage in joints. This breakthrough has the potential to transform the lives of millions, restoring mobility and improving quality of life.
Imagine a world where individuals suffering from joint pain due to cartilage damage can find relief through regenerative medicine. Stem cell-based therapies offer a promising alternative to traditional treatments, such as invasive surgeries or long-term pain management. With the ability to grow cartilage in a laboratory, doctors could provide patients with a long-lasting solution that not only alleviates pain but also restores joint function, allowing individuals to regain their independence and engage in activities they once thought were impossible.
Athletes and sports enthusiasts often face injuries that require specialized care. The ability to grow cartilage from stem cells could pave the way for advanced treatment options in sports medicine, allowing athletes to recover faster and regain peak performance.
Consider a professional athlete who sustains a career-threatening knee injury. In the past, their road to recovery might have been uncertain, with the possibility of never returning to their previous level of performance. However, with the advent of stem cell-based therapies, the athlete's outlook changes dramatically. By utilizing stem cells to grow new cartilage, their damaged joint can be repaired with precision and efficiency. This innovative approach not only accelerates the healing process but also enhances the athlete's chances of making a successful comeback, ensuring that they can continue to inspire and entertain fans around the world.
Looking forward, the research conducted at TU is just the beginning of a transformative journey in stem cell science.
The dedicated team at TU is committed to pushing the boundaries of what is possible with stem cells. Their next steps involve further refining the techniques used in cartilage growth, expanding their understanding of stem cell differentiation, and exploring the potential of combining stem cells with other regenerative therapies.
While the focus of this research is on cartilage growth, the applications of stem cell research extend far beyond this realm. Stem cells hold promise in treating a variety of conditions, from neurological disorders to heart disease. Their potential to revolutionize the field of medicine is truly extraordinary.
Alongside the scientific progress, stem cell research brings with it important ethical considerations. The use of embryonic stem cells, in particular, has sparked heated debates. These ethical considerations must be carefully addressed to ensure responsible and transparent research practices.
In conclusion, researchers at TU are making remarkable strides in growing cartilage from stem cells, offering hope for customized injury repair. This breakthrough has the potential to transform the field of injury treatment and rehabilitation, revolutionizing the way we approach injuries in the future. As we stand on the brink of a new era in medicine, stem cell research holds the key to unlocking a world where injuries are no longer seen as insurmountable obstacles, but as opportunities for personalized healing.